Why Clinical Trials Matter
Clinical trials are a cornerstone of medical research. They provide the scientific evidence needed to bring new treatments to market and ensure that they are safe and effective. Clinical trials are research studies conducted with human participants to evaluate the safety and effectiveness of new medical treatments, interventions, or devices. These trials follow a strict protocol to ensure the reliability of the results and the safety of the participants.
Get Involved
Participation in clinical trials is voluntary, but your involvement can make a significant impact. By joining a trial, you can help accelerate the development of new treatments and contribute to the hope of a brighter future for everyone affected by PWS.
Why Participate in Clinical Trials?
Participation in clinical trials offers several benefits:
- Access to New Treatments: Participants may gain access to new treatments before they are widely available.
- Contributing to Research: By participating, individuals help advance medical research and contribute to finding new therapies for PWS.
- Comprehensive Care: Participants receive close monitoring and care from medical professionals throughout the trial.
The Phases of Clinical Trials
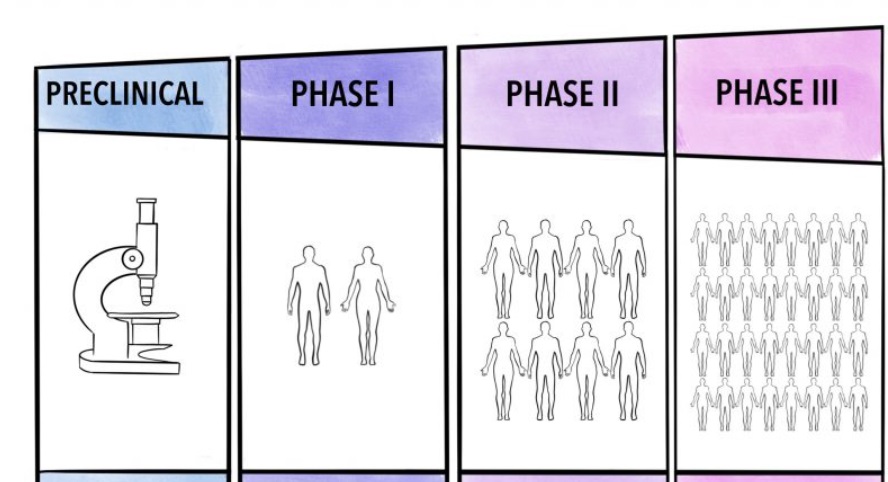
Clinical trials typically proceed through several phases, each with a specific purpose:
- Phase I – Safety and Dosage:
- Small group of participants
- Focuses on evaluating the safety, determining a safe dosage range, and identifying side effects
- Phase II – Efficacy and Side Effects:
- Larger group of participants
- Assesses the effectiveness of the treatment and further evaluates its safety
- Phase III – Confirmation and Comparison:
- Even larger group of participants
- Confirms the treatment’s effectiveness, monitors side effects, and compares it to commonly used treatments
- Post-Marketing Surveillance:
- Conducted after the treatment has been approved for public use
- Continues to monitor the treatment’s long-term effects and effectiveness in a broader population
Steps Involved in a Clinical Trial
- Pre-Clinical Research: Before a treatment reaches the clinical trial stage, it undergoes extensive laboratory testing and studies to gather initial data on its safety and potential effectiveness.
- Study Design and Approval: Researchers develop a detailed plan (protocol) for the trial, which must be reviewed and approved by regulatory authorities and ethics committees to ensure it meets all safety and ethical standards.
- Recruitment: Participants are recruited based on specific criteria outlined in the protocol. This ensures that the study includes individuals who are most likely to benefit from the treatment and that the results will be reliable.
- Informed Consent: Potential participants are provided with comprehensive information about the trial, including its purpose, procedures, risks, and benefits. They must give their informed consent before joining the study.
- Conducting the Trial: The treatment is administered to participants according to the protocol. Researchers closely monitor the participants and collect data on their health and response to the treatment.
- Data Analysis: After the trial is completed, researchers analyse the collected data to determine the treatment’s safety and effectiveness. The results are then reviewed by regulatory authorities.
- Approval and Monitoring: If the treatment is found to be safe and effective, it may be approved for public use. Even after approval, ongoing monitoring continues to ensure its safety in the general population.
Stay Updated
We regularly update our website with new trials and important announcements. Bookmark this page and check back often to stay informed about the latest advancements in PWS research.
Together, we can drive progress and bring hope to those living with Prader-Willi Syndrome. Thank you for your support and commitment to our mission.
For more information or to get involved, please contact us at info@fpwr.org.uk
